Identification of Patients At-Risk of QT Interval Prolongation during Medication Reviews: A Missed Opportunity?
J. Clin. Med. 2018, 7(12), 533; doi:
Article
1
Faculty of Health, University of Canberra, Bruce, ACT 2617, Australia
2
School of Medicine, University of Tasmania, Sandy Bay, TAS 7005, Australia
*
Author to whom correspondence should be addressed.
Received: 7 November 2018 / Accepted: 7 December 2018 / Published: 10 December 2018
Abstract
:The prolongation of the QT interval is a relatively rare but serious adverse drug reaction. It can lead to torsade de pointes, which is potentially life-threatening. The study's objectives were: determine the use of QT interval-prolonging drugs in an elderly community-dwelling population at risk of medication misadventure and identify recommendations regarding the risk of QT interval prolongation made by pharmacists when performing medication reviews. In a retrospective evaluation, 500 medication review reports from Australian pharmacists were analysed. In patients taking at least one QT interval-prolonging drug, the individual risk of drug-induced QT interval prolongation was assessed. Recommendations of pharmacists to avoid the occurrence of this drug-related problem were examined. There was a high prevalence of use of potentially QT interval-prolonging drugs (71% patients), with 11% of patients taking at least one drug with a known risk. Pharmacists provided specific recommendations in only eight out of 35 patients (23%) with a high-risk score and taking drugs with known risk of QT interval prolongation. Pharmacists' recommendations, when present, were focused on drugs with known risk of QT interval prolongation, rather than patients' additional risk factors. There is a need to improve knowledge and awareness of this topic among pharmacists performing medication reviews.
Keywords:
QT interval prolongation; torsade de pointes; medication review; pharmacist intervention1. Introduction
This study explored the role of pharmacists in identifying and reducing the risk of drug-induced QT interval prolongation when conducting medication reviews. The aim was to determine the prevalence of use of QT interval-prolonging drugs in an elderly community-dwelling population at risk of medication misadventure and to identify recommendations made by accredited pharmacists regarding QT interval prolongation in medication review reports.
2. Experimental Section
3. Results
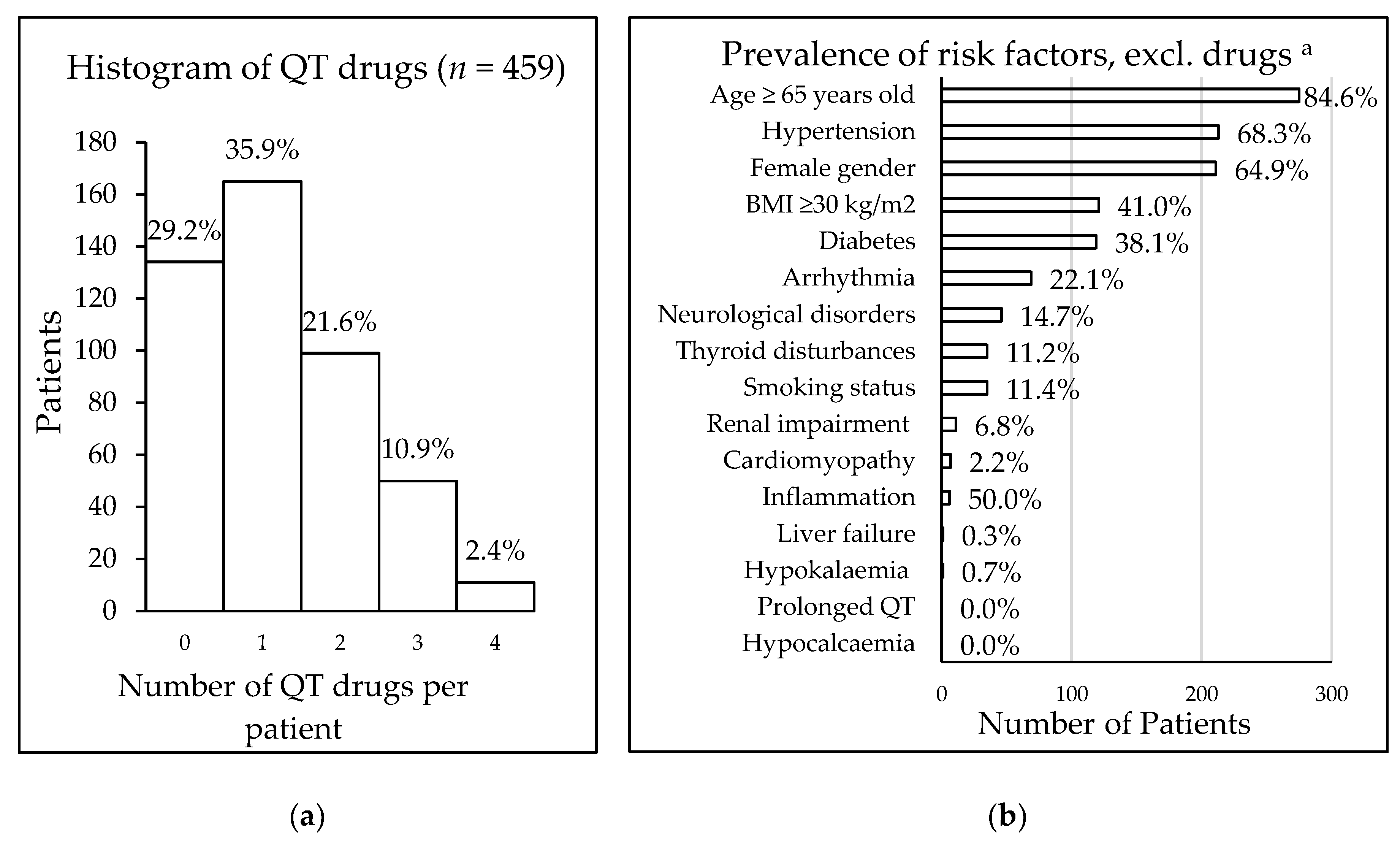
Figure 1. (a) Frequency of patients taking QT interval-prolonging drugs; (b) prevalence of additional risk factors for 325 patients taking QT interval-prolonging drugs, a data availability: age and gender n = 325, medical history n = 312, smoking n = 306, BMI n = 295, renal status n = 161, potassium level n = 151, calcium level n = 13, inflammation (CRP level) n = 12. Abbreviation: BMI = body mass index.
Table 3. Baseline characteristics of patients taking QT interval-prolonging drugs (n = 325) and commonly taken QT interval-prolonging medication (n = 459).
Out of 35 patients with a high-risk RISQ-PATH score of ≥10 and taking drugs having a known risk of QT interval prolongation, pharmacists provided specific recommendations regarding QT interval prolongation in only eight cases (23%). In a further eight cases (23%) they provided unspecific advice (e.g., risk of interaction, not suitable for patient, or recommended dose reduction), in six cases (17%) they mentioned a different potential adverse effect of the drug, and in the remaining 13 cases (37%) the pharmacists did not make any recommendation. It should be noted that donepezil was added to the CredibleMeds® website in March 2015. That means that these results included one medication review report with unspecific advice regarding donepezil and two reports without any recommendations regarding donepezil, that were written before the drug was listed as "known risk".
Overall, in 15 of 325 HMR reports (4.6%) for patients taking potential QT interval-prolonging drugs, the pharmacists specifically mentioned patients being at risk of QT interval prolongation. The comments were regarding excessive doses of specific drugs (citalopram n = 2, escitalopram n = 2, domperidone n = 2), prescription of specific drugs (tricyclic antidepressants n = 5, sotalol n = 1), and pharmacotherapy with several QT interval-prolonging drugs concomitantly (n = 9); some recommendations comprised several of these aspects. The suggested interventions were monitoring patients closely (n = 5), monitoring electrolytes (n = 2), change of drug (n = 4), withdrawal (n = 4), dose reduction (n = 4), and specialist referral (n = 3); for some patients several interventions were suggested. The median risk score for QT interval prolongation in these 15 patients was 11.25 (ranging from 1.75 to 16.25).
4. Discussion
This study had a few limitations. Since the study was retrospective, the research team was not able to check the validity of the recorded data. In some HMR reports, data for the risk calculation was not available, especially regarding the pathology data. That might have led to an underestimation of the prevalence of risk factors (e.g., electrolyte imbalances or co-morbidities) since patients without the recorded data were categorized as not having the risk factor. The CredibleMeds® drug list is updated when new evidence regarding a drug's potential to induce QT interval prolongation is identified. The reports included in this study were written over a period of four years. This means that at the point of the medication review, a drug might not have been listed. This limitation was minimised by using a list with status as of 2015.
5. Conclusions
There is an appreciable risk of drug-induced QT interval prolongation among patients undergoing medication reviews. Furthermore, the presence of other risk factors, such as older age and gender, puts many patients at additional risk of this adverse event. Pharmacists' recommendations in medication reviews, when present, were targeted on drugs with known risk of QT interval prolongation, rather than patients' additional risk factors. There is a need to improve knowledge and awareness of this topic amongst pharmacists performing medication reviews.
Author Contributions
Conceptualization, V.H.B., K.L., and S.K.; Methodology, V.H.B., M.N., G.M.P., and S.K.; Formal analysis, V.H.B., M.N., G.M.P., and S.K.; Investigation, V.H.B., and K.L.; Resources, S.K.; Data curation, V.H.B.; Validation, V.H.B., M.N., G.M.P., and S.K.; Writing—Original draft preparation, K.L. and V.H.B.; Writing—Review and editing, M.N., G.M.P., and S.K.; Visualization, V.H.B.; Supervision, M.N., and S.K.; Project administration, S.K.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yap, Y.G.; Camm, A.J. Drug induced QT prolongation and torsades de pointes. Heart 2003, 89, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- The Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhytmic Potential for Non-Antiarrhytmic Drugs E14. Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf (accessed on 10 January 2018).
- Isbister, G.K.; Page, C.B. Drug induced QT prolongation: The measurement and assessment of the QT interval in clinical practice. Br. J. Clin. Pharmacol. 2013, 76, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Trinkley, K.E.; Lee Page, R.; Lien, H.; Yamanouye, K.; Tisdale, J.E. QT interval prolongation and the risk of torsades de pointes: Essentials for clinicians. Curr. Med. Res. Opin. 2013, 29, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Zeltser, D.; Justo, D.; Halkin, A.; Prokhorov, V.; Heller, K.; Viskin, S. Torsade de Pointes due to noncardiac drugs: Most patients have easily identifiable risk factors. Medicine 2003, 82, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, J.J.; Wafer, M.; Fitzgerald, G.; Nawaz, A.; O'Brien, C.; Liston, R. QTc prolongation in acute medical admissions: An often overlooked and potentially serious finding. Postgrad. Med. J. 2018, 94, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, J.E. Drug-induced QT interval prolongation and torsades de pointes: Role of the pharmacist in risk assessment, prevention and management. Can. Pharm J. (Ott) 2016, 149, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Pharmaceutical Society of Australia. Guidelines for Pharmacists Providing Home Medicines Review (HMR) Services; Canberra, ACT, Australia, 2011; pp. 1–20. [Google Scholar]
- Ahn, J.; Park, J.E.; Anthony, C.; Burke, M. Understanding, benefits and difficulties of home medicines review—Patients' perspectives. Aust. Fam. Physician 2015, 44, 249–253. [Google Scholar] [PubMed]
- CredibleMeds, QTdrugs List. Available online: www.CredibleMeds.org (accessed on 10 January 2018).
- Vandael, E.; Vandenberk, B.; Vandenberghe, J.; Willems, R.; Foulon, V. Risk factors for QTc-prolongation: Systematic review of the evidence. Int. J. Clin. Pharm. 2017, 39, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Vandael, E.; Vandenberk, B.; Vandenberghe, J.; Spriet, I.; Willems, R.; Foulon, V. Development of a risk score for QTc-prolongation: The RISQ-PATH study. Int. J. Clin. Pharm. 2017, 39, 424–432. [Google Scholar] [CrossRef] [PubMed]
- LaPointe, N.M.A.; Al-Khatib, S.M.; Kramer, J.M.; Califf, R.M. Knowledge deficits related to the QT interval could affect patient safety. Ann. Noninvasive Electrocardiol. 2003, 8, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; LaPointe, N.M.A.; Kramer, J.M.; Chen, A.Y.; Hammill, B.G.; Delong, L.; Califf, R.M. A survey of health care practitioners' knowledge of the QT interval. J. Gen. Intern. Med. 2005, 20, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Vandael, E.; Verstuyft, E.; Leirs, C.; Foulon, V. An e-learning programme about the risk and management of QTc-prolongation in community pharmacies significantly improves pharmacists' (long-term) knowledge. Pharm. Educ. 2018, 18, 119–131. [Google Scholar]
- Ng, T.M.; Bell, A.M.; Hong, C.; Hara, J.M.; Touchette, D.R.; Danskey, K.N.; Lindsay, T.T.; Puumala, S.E. Pharmacist monitoring of QTc interval–prolonging medications in critically ill medical patients: A pilot study. Ann. Pharmacother. 2008, 42, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, L.M.; Temple, J.D.; Hilmas, E. Impact of pharmacist intervention on electrocardiogram monitoring of pediatric patients on multiple QTc interval-prolonging medications. J. Pediatr. Pharmacol. Ther. 2017, 22, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Dhanani, T.C.; Mantovani, E.H.; Turner, J.R. Clinical pharmacists' opportunities to reduce inappropriate prescription of QT-prolonging medications: Calls to action. Int. J. Pharm. Pract. 2017, 25, 176–179. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).


No hay comentarios:
Publicar un comentario
Danos tu opinion, enriquece el post.